Advancing synaptic imaging: the Blanpied Lab’s approach
Understanding brain function, particularly at the level of neuronal synapses, is a complex and crucial endeavor in neuroscience.
Neuronal synapses are where many neurological processes occur, including signal transmission and plasticity, which underlie learning and memory. Imaging these tiny structures presents significant challenges due to their minuscule size, often requiring advanced microscopy techniques for adequate resolution.
The lab of Thomas Blanpied at the University of Maryland School of Medicine is at the forefront of this research. They employ a variety of cutting-edge microscopy methods to explore the intricacies of synaptic active zones and the functions of the postsynaptic density.
In particular, they are using PALM (Photoactivated Localization Microscopy) and STORM (Stochastic Optical Reconstruction Microscopy) single-molecule super-resolution microscopy techniques to overcome the diffraction limit of conventional light microscopy and visualize neuronal structures at the nanometer scale.
Both techniques rely on the precise localization of single molecules that are activated and deactivated stochastically. By compiling the positions of these molecules, researchers can construct a super-resolved image.
Improving image resolution: the role of Adaptive Optics in synaptic imaging
The localization precision of molecule positions in super-resolution microscopy is highly dependent on the number of detected photons and the quality of single-molecule spots, also known as the point spread function (PSF). In neuronal samples, differences in refractive indices between different cellular components can cause optical aberrations. These aberrations distort the PSF, leading to reduced localization precision and image resolution.
To overcome aberrations and restore image resolution, scientists in the Blanpied lab use an adaptive optics device called MicAO 3DSR. This device features a deformable mirror capable of compensating for aberrations, correcting the PSF, and thereby improving the resolution of super-resolved images.
How to use advanced imaging techniques, an example:
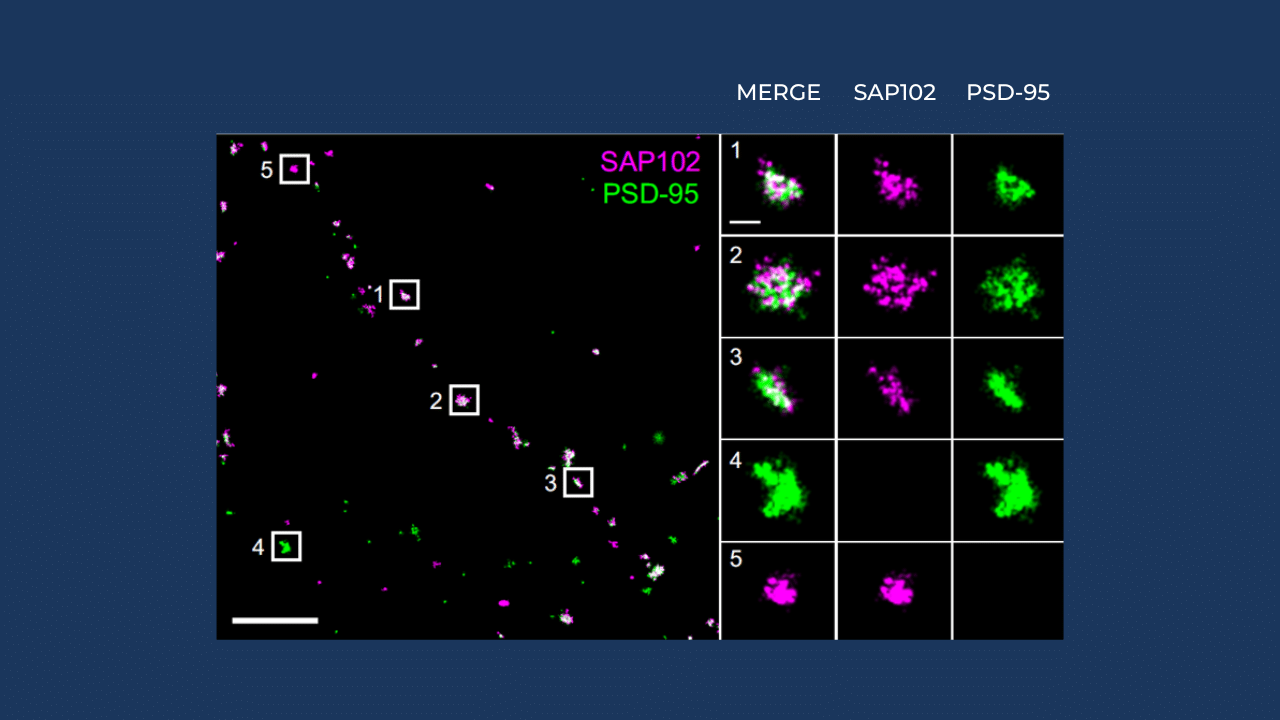
An example of such imaging was recently published in the Journal of Neuroscience: “Distinct SAP102 and PSD-95 Nano-Organization Defines Multiple Types of Synaptic Scaffold Protein Domains at Single Synapses”. This study investigates the nano-organization of two key synaptic scaffold proteins, SAP102 and PSD-95, within individual synapses.
By employing super-resolution microscopy enhanced with adaptive optics, the researchers were able to:
- Visualize spatial distribution: Precisely map the localization of SAP102 and PSD-95 at single synapses.
- Identify multiple domains: Discover multiple types of scaffold protein domains, indicating a complex and heterogeneous organization within synapses.
- Provide insights into synaptic function: Offer new understanding of how different scaffold protein organizations may contribute to synaptic function and plasticity.
Significance and Impact of Blanpied Lab’s work
Integration of adaptive optics with super-resolution microscopy in the Blanpied lab enhances the ability to study the fine details of neuronal synapses. The use of devices like MicAO 3DSR to correct aberrations and improve PSF quality is crucial for advancing our understanding of brain function and dysfunction. These technological advancements enable scientists to:
- Achieve high precision: Overcome challenges posed by refractive index mismatches, resulting in highly precise localization of molecules within synapses.
- Visualize dynamic processes: Capture real-time changes in protein organization, allowing for a deeper understanding of synaptic dynamics and plasticity.
- Inform neurological research: Provide crucial insights into the molecular mechanisms underlying synaptic function and their alterations in disease states.
